As we discussed in the first installment of this particular editorial endeavor for the Best Food Importers website dedicated to the overall presentation of the the general food labeling requirements, a new set of legislative impositions came into effect at the end of the last year through the new Regulation (EU) No. 1169/2011. This regulation came in effect beginning with 13th of December 2014, and, as such, is still of great actuality for any food producer that wants to gain access to the European Union food market, whatever the product itself may be. The previous installment of this multi-part article focused on font, largest surface area and name of food aspects of the wider general food labeling topic.
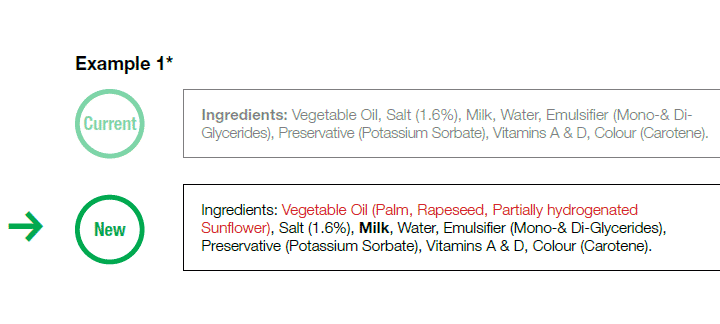
Now, we shall shift our attention towards two new topics that were affected by the new changes introduced by the new Regulation (EU) No. 1169/2011, regulation that came into effect at the end of last year, namely the allergen labeling and the ingredient listings. There are few changes introduced by the new regulations in regards to ingredient listings, and these changes target specific products. First of all, engineered nano-materials must be labeled as such. Furthermore, the generic term ‘Vegetable Oil/Fat’ is no longer permitted in the list of ingredients; the specific name of the oil/fat must be declared, as in the following example.
And lastly, carbonated energy drinks with caffeine must contain the following warning:

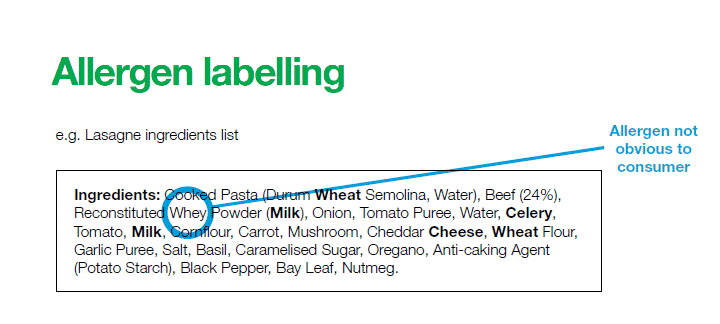
Allergens must be emphasized in ingredient list, each occurrence, by means of font, style or background color, e.g. bold, but only the name of the substance or product as listed in Annex II of the respective regulation shall be emphasized.
Any allergenic processing aid still present in the finished product (even if in an altered form) needs to be listed and highlighted. Exempted are the cases in which the products are characterized by the absence of ingredients list (e.g. alcohol) – must say “contains” and the name of the allergen.Allergen boxes are no longer adequate; however, they may be used to direct consumers to the allergens highlighted in the ingredient declaration e.g. “Allergy Advice: For allergens, see ingredients in bold.” Indication of allergens is extended to non-prepacked food; allergens must be available in easily visible written format unless national rules are adopted. The Department of Health intend to introduce such rules.
Image courtesy of VFS Digital Design – Some rights reserved
© www.bestfoodimporters.com 2014